The Chemistry Paper 1 is a multiple choice paper that makes up 20% of your overall chemistry grade. The maximum marks that you can receive is 30 for SL and 40 for HL and you will only have 45 mins (SL) or 1 hour (HL) to complete. That means that you should only spent about 1 minute and 30 seconds per question, which is not a lot of time. So, read the tips below to brush up your exam skills so that you can save time!
1. Write Out the Formulas to Avoid Confusion
Even if you know your formulas inside out, it is always good to write them out when double checking your answers to make sure that everything is done correctly. For those who are struggling for Unit 1, this is one of the things that you should always do because it will help you identify which values you should use from the questions and how to use them.
Ex. How many molecules are present in a drop of methanol, CH3OH, of mass 1.6 × 10-3 g? ( L= 6.0 × 1023 mol-1)
A. 6.0 × 1019
B. 6.0 × 1026
C. 3.0 × 1019
D. 3.0 × 1020
① Write down the formulas needed for this question:
- n = m / M
M: molar mass, m: mass of a substance in grams, n: number of moles of a substance
- n × L= number of molecules
n: number of moles of a substance, L: Avogadro’s constant (6.0 × 1023 mol-1)
② Plot the values into these formulas and start calculating:
- The mass (m) of methanol: 1.6 × 10-3 g
- The molar mass (M) of methanol: 32 g mol-1
- Avogadro’s constant (L): 6.0 × 1023 mol-1
Here is how your workout would look like during your exam when you use this technique:
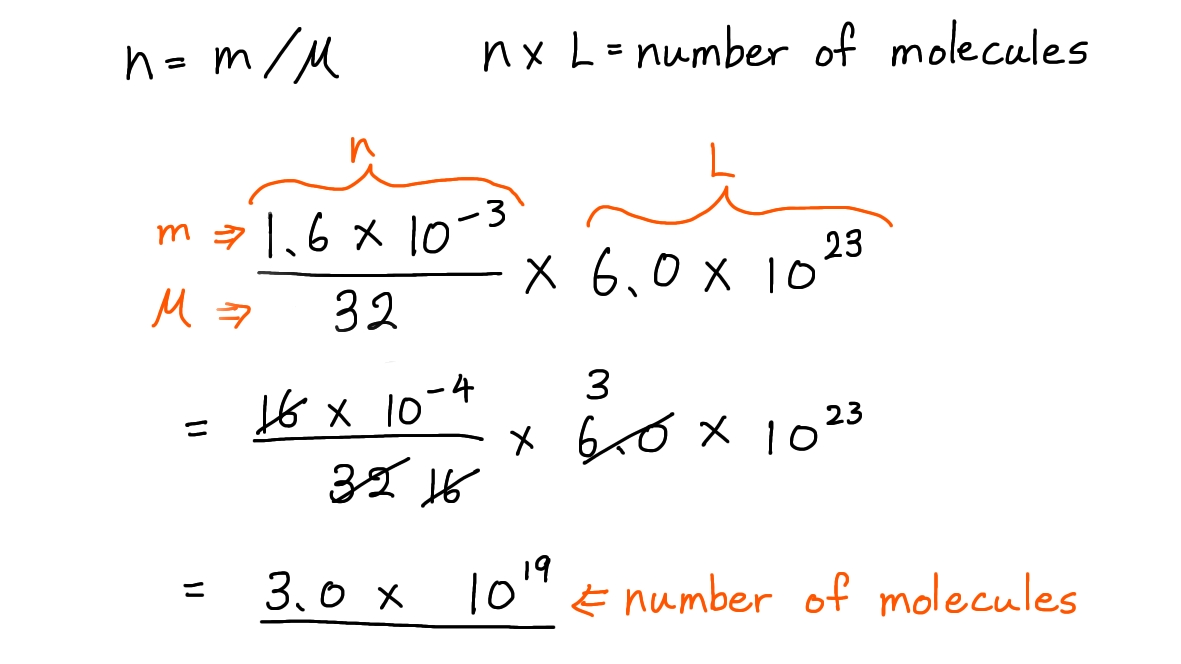
Hence, the correct answer is C.
2. Draw the Bonding Structures
Now, the entire Unit 4 is about bonding and you will be asked to identify delocalised electrons, sigma/ pi bonds, lone pairs, and shapes of the molecules or compounds. Know how to draw these structures properly and use the periodic table to help you navigate through the process whenever you are stuck. The periodic table tells you how many electrons each element has so use this tool!
Ex. For the following molecules, which one has a non-bonding pair of electrons on the central atom?
A. CH4
B. BF3
C. SO2
D. MgF2
① Draw the Lewis structures for these molecules.
② Identify the answer by looking at the structures.
Here is an example of what you should draw in this situation:
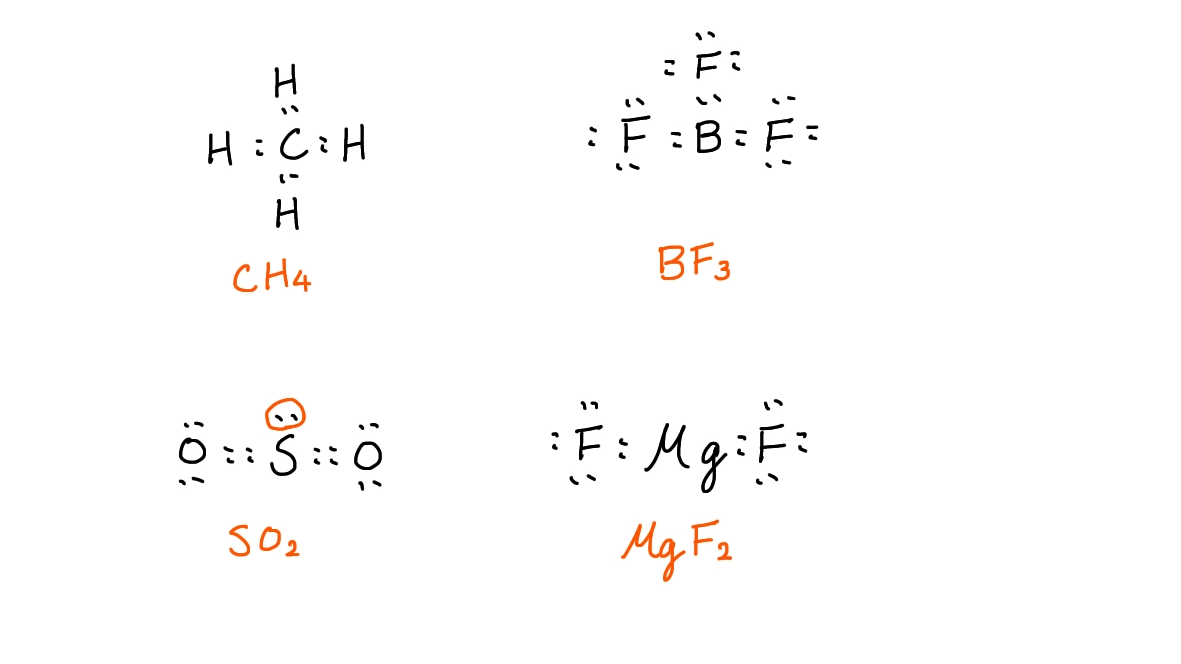
By looking at these structures, we can see that SO2 is the only molecule that has a non-binding pair of electrons.
Hence, the correct answer is C.
3. Use the Elimination Technique
The questions on the exam can get pretty wordy. If you read the answers carefully, you will realise that some of them contain unnecessary information to trick you on purpose. Don’t be fooled! Trust yourself and eliminate anything that doesn’t feel right or sound right. This technique is very effective when you can’t pick one correct answer at your first glance. Don’t panic. Try to eliminate two to three answers. That way, you will be able to see which one is the correct answer.
Ex. Which of the following statements about the periodic table are correct?
I. Elements in group 1 show a gradual change in physical properties.
II. The atomic radii of the elements increases across period 4.
III. The electronegativity of the elements increase across period 3.
A. I and II only
B. I and III only
C. II and III only
D. I, II, and III
① Read through the options from the top and eliminate the answers when you see something that is incorrect.
② Double check your final answer just to make sure that it is the correct option left after the eliminations.
Here is how you would go through this process:

- Option C can be eliminated right away as statement I is correct.
- Because the atomic radii of the elements “decreases” rather and increases across period 4, statement II is incorrect.
- Therefore, we can eliminate A and D
- This means that the final answer should be B as it is the only option left.
- However, you should always double check the last statement just in case you have misread some of the previous statements.
Hence, the correct answer is B.
4. Write Out the Chemical Equations When Needed
Sometimes, you might have to write out the entire chemical equation in order to understand what is going on and what kind of information the question is asking for. Whenever you don’t understand the question, try writing out a chemical equation that sums up the entire question. That way, you can see which values are in blank, which tells you what kind of calculations you will have to do in order to fill in those gaps.
Ex. When 5.00g of ethene (C2H4, Mr=28.1) undergoes complete combustion, what volume of carbon dioxide, in dm3 under standard conditions, is formed?
A. (22.4 × 28.1)/ 5.00
B. (22.4 × 5.00)/ 28.1
C. (2 × 22.4 × 28.1)/ 5.00
D. (2 × 22.4 × 5.00)/ 28.1
① Write down the chemical equation of this reaction and balance it:
C2H4 + 3O2 → 2CO2 + 2H2O
② Write down the formulas needed for this question:
- n = m / M
M: molar mass, m: mass of a substance in grams, n: number of moles of a substance
- n × 22.4 (L) = L
n: number of moles of a substance, 22.4: molar volume at standard conditions, L: volume of gas
③ Plot the values into these formulas:
- The mass (m) of ethene: 5.00g
- The molar mass (M) of methanol: 28.1 g mol-1
- Molar volume at standard conditions (L): 22.4 L mol-1
Here is how your workout would look like during your exam when you use this technique:
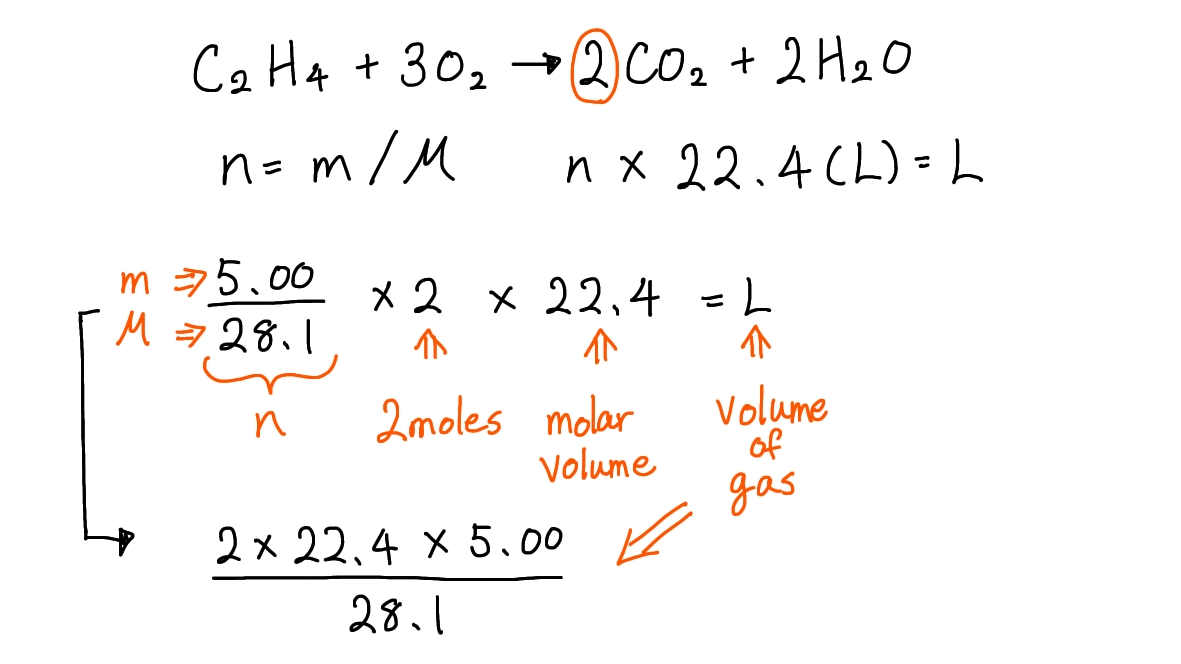
Hence, the correct answer is D.
5. Familiarise Yourself With the Calculations
There are a certain amount of questions that require you to do calculations. Remember, you are not allowed to use the calculator for this paper so make sure that you can do everything by hand. Don’t worry, there won’t be any overly complicated values for paper 1. Practice your calculation skills so that you won’t be wasting time on these questions. If you can’t get the answer after two attempts, skip it first and come back at the end!
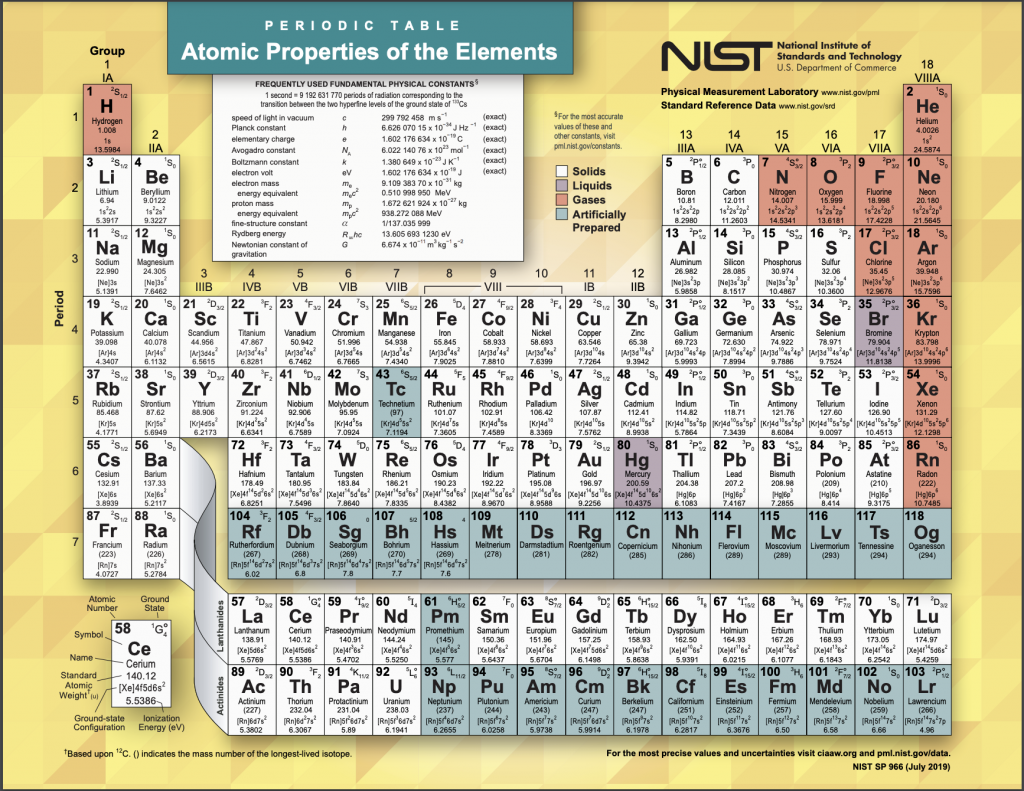
6. Know the Periodic Table
As mentioned previously, the periodic table can actually help you a lot when it comes to drawing the structures or identifying the molar masses. It is also the only tool that you are allowed to use in this exam so use it wisely. Know which element is in which column or row so that you won’t waste time on trying to find one single element for the exam. Also, know where the s-block, p–block, d-block, f-block, and transition elements are, as well as the periodic trends.